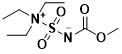
Burgess reagent CAS 29684-56-8
Specification
|
ITEM |
STANDARD |
|
Appearance |
White to light yellow solid |
|
Purity (HPLC) |
≥ 95% |
|
NMR |
Conforms to structure |
|
Melting point |
76-79 °C |
Application
Burgess dehydrating agent, also called Burgess reagent, is methyl N-(triethylaminosulfuryl) carbamate. It can be dehydrated under mild conditions and is a mild selective dehydrating agent. It is used to convert secondary and tertiary alcohols with adjacent protons to alkenes.
It can be prepared by the reaction of chlorosulfone isocyanate and triethylamine in methanol. During dehydration, sulfur is attacked by hydroxyl group, and then cis elimination occurs. Burgess dehydrating agents can also be used to synthesize isonitrile compounds from formamide. The reaction mechanism is similar to the xanthate elimination reaction, and the alkene is also obtained by cis elimination of the transition state of the six-membered ring in the molecule.
Packing & Storage
100g/500g/1kg/25kg or as request;
Non-hazardous chemicals, vacuumed storage at low temperature (2-8°C).
(Special packaging, no deactivation. No cryogenic transport is required.)







